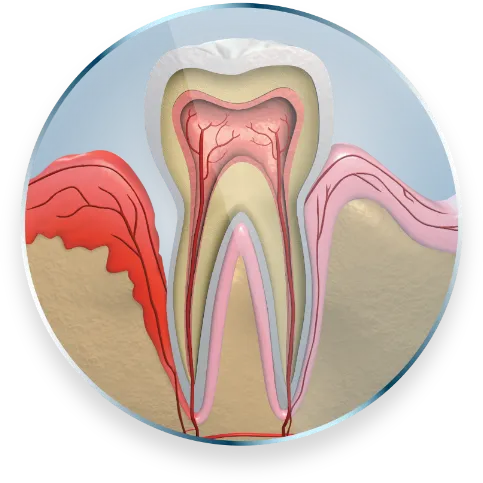
Demonstrated statistically significant pocket depth (PD) reduction at 9 months1
Choose ARESTIN in combination with SRP for sustained results.1
When incorporated into a routine periodontal
maintenance program, ARESTIN + SRP
Demonstrated consistent reduction at
9 months1
Reduced PD by nearly
20% more
than SRP alone in patients with a ≥2 mm PD reduction1
Was nearly
3 times
more likely to reduce mean PD than SRP alone, from ≥6 mm to <5 mm1
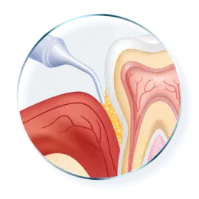
Active in the pocket for at least 14 days1
SELECT IMPORTANT SAFETY INFORMATION
- ARESTIN is contraindicated in any patient who has a known sensitivity to minocycline or tetracyclines.

Demonstrated statistically significant reduction in pocket depth (PD) at 9 months1
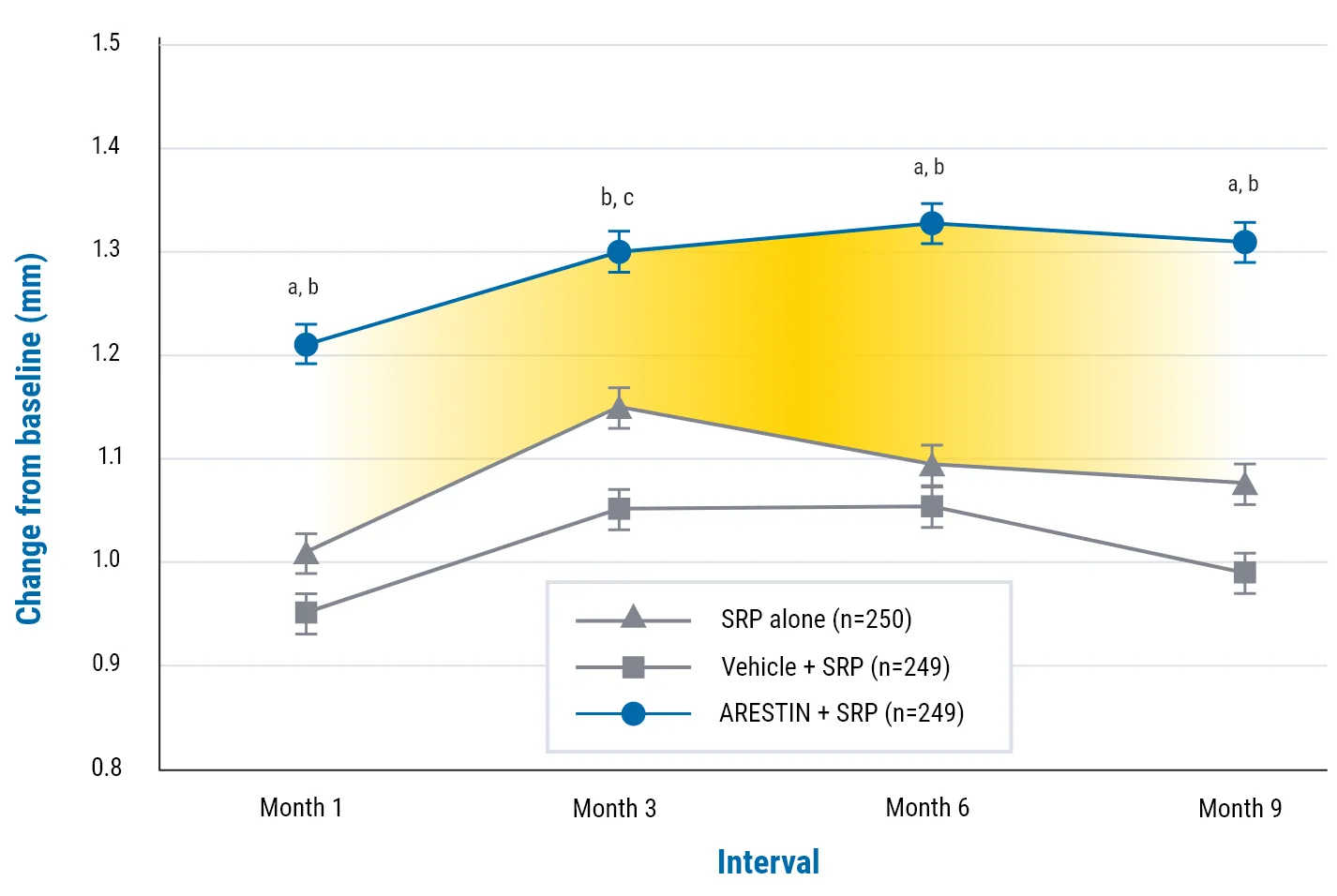
aP <0.001, ARESTIN + SRP vs SRP alone.
bP <0.001, ARESTIN + SRP vs vehicle.
cP <0.01, ARESTIN + SRP vs SRP alone.
NOTE: Average PD reduction (adjusted means) from baseline of >5 mm. The difference in mean PD reduction at 9 months was 0.24 mm.1

In 2 well-controlled, multicenter, investigator-blind, vehicle-controlled, parallel-design studies (3 arms) of 748 subjects (study OPI-103A=368, study OPI-103B=380) with generalized moderate-to-advanced adult periodontitis characterized by mean probing depths of 5.90 and 5.81 mm, respectively, were enrolled. In these 2 studies, an average of 29.5 (5-114), 31.7 (4-137), and 31 (5-108) sites were treated at baseline in the SRP alone, SRP + vehicle, and SRP + ARESTIN groups, respectively. When these studies were combined, the mean pocket depth reductions at 9 months were 1.18 mm, 1.10 mm, and 1.42 mm for SRP alone, SRP + vehicle, and SRP + ARESTIN, respectively.

Every millimeter matters
40.5% of patients
in the ARESTIN + SRP group achieved PD reduction at 9 months compared with 32.87% and 28.98% of patients treated with SRP alone and SRP + vehicle, respectively1

In a subgroup of patients with more advanced disease (baseline mean PD ≥6 mm), reduction in bleeding on probing was:
26.04%
for ARESTIN + SRP1
19.80%
for SRP alone1
18.11%
for SRP + vehicle1
SELECT IMPORTANT SAFETY INFORMATION
- The use of drugs of the tetracycline class during tooth development may cause permanent discoloration of the teeth, and therefore should not be used in children or in pregnant or nursing women.
In 2 well-controlled, multicenter, investigator-blind, vehicle-controlled, parallel-design studies (3 arms) of 748 subjects (study OPI-103A=368, study OPI-103B=380) with generalized moderate-to-advanced adult periodontitis characterized by mean probing depths of 5.90 and 5.81 mm, respectively, were enrolled. In these 2 studies, an average of 29.5 (5-114), 31.7 (4-137), and 31 (5-108) sites were treated at baseline in the SRP alone, SRP + vehicle, and SRP + ARESTIN groups, respectively. When these studies were combined, the mean pocket depth reductions at 9 months were 1.18 mm, 1.10 mm, and 1.42 mm for SRP alone, SRP + vehicle, and SRP + ARESTIN, respectively.



Additional data from a randomized, open label, controlled, prospective clinical trial of individuals without comorbidities3:
ARESTIN + SRP produced larger decrease in clinical markers of periodontitis than SRP alone3
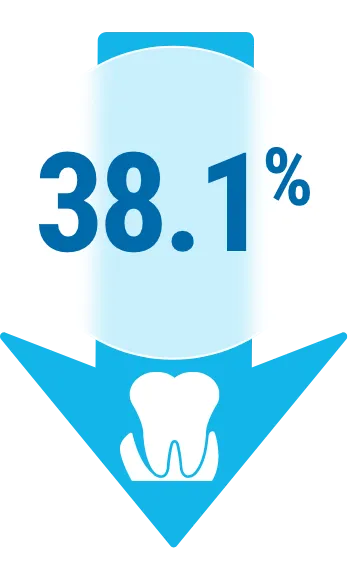
POCKET DEPTH (PD)
PD decreased by a mean of 38.1%, from 5.4 mm at baseline to 3.34 mm at 6 months.3

BLEEDING ON PROBING (BOP)
BOP decreased by a mean of
84.4%, from 48.8% at baseline to
7.6% at 6 months.3
Limitations included the lack of a blinded examiner for clinical outcomes and the lack of patient-reported outcome measures.3
SELECT IMPORTANT SAFETY INFORMATION
- Tetracyclines, including oral minocycline, have been associated with development of autoimmune syndromes including a lupus-like syndrome manifested by arthralgia, myalgia, rash, and swelling.
ARESTIN was studied in a randomized, open-label, controlled clinical trial comparing ARESTIN + SRP vs SRP alone. Subjects were 70 male and female adults ≥18 years old with a diagnosis of Stage II to Stage IV, Grade B periodontitis. Inclusion criteria were a minimum of 8 sites with a PD of ≥5 mm and 8 sites of BOP in any of the 4 quadrants. The primary outcome measure was an assessment of the adjunctive effects of ARESTIN on PD, CAL, BOP, and GI compared with SRP alone. The secondary outcome measure was a determination of the relative numbers of 11 periodontal pathogens in saliva after treatment with ARESTIN + SRP compared with SRP alone.




“Superiority for the SRP plus minocycline microsphere group was maintained throughout the study… The difference between SRP plus minocycline microspheres and the other groups after 9 months was statistically significant at P <0.001.”1
Additional data from a randomized, parallel-group clinical trial of individuals with moderate-to-advanced chronic periodontitis4:
ARESTIN + SRP produced a greater mean reduction in the proportion of red complex bacteria (RCB) at 30 days compared with SRP alone4
24%
greater decrease
In patients with an RCB reduction of ≥50%, there was a 24% greater decrease for ARESTIN + SRP compared with SRP alone (40.3% vs 30.8%, respectively)4
7%
ARESTIN + SRP
10%
SRP alone
RCB in
periodontal biofilm
At 30 days, the periodontal biofilm showed evidence of fewer RCBs in the ARESTIN + SRP group vs SRP alone4
The microbiological goal of periodontal therapy is to lower periodontal pathogens to levels seen in healthy patients (approximately 7% RCBs)4
SELECT IMPORTANT SAFETY INFORMATION
- The use of ARESTIN in an acutely abscessed periodontal pocket or for use in the regeneration of alveolar bone has not been studied.
Study Limitations4
Bacterial measures taken from 5 investigators at 5 different sites are bound to be variable. Improvements in these measures may be made by averaging across each patient’s periodontal treatment sites by dividing by the estimated total number of bacteria to obtain proportions. Such normalization procedures were used to reduce the variability in numbers of bacteria in a given sample.
Multicenter, single-blind, randomized, parallel-group study of 127 subjects with moderate-to-advanced chronic periodontitis who had at least five teeth with probing depths (PDs) ≥5 mm (test sites). For the primary endpoints, subjects treated with MM + SRP achieved a significantly greater mean reduction in the proportion of red complex bacteria (RCBs) at 30 days compared to those treated with SRP alone (6.5% versus 5.0%, respectively; P=0.0005). For each category of percent reduction in RCB proportions from baseline, subjects treated with MM + SRP achieved a reduction of ~5% greater than that with SRP alone. However, this difference was twice as large in subjects achieving the highest reduction in RCB number (≥50%).




“Minocycline microsphere therapy worked similarly in men and women, and at 9 months reached statistical significance in each (P ≤0.004). Probing depth reduction was significantly greater for the SRP plus minocycline microsphere group at all examinations.”1

Additional data from a randomized, open label, controlled, prospective clinical trial:
Decreases in the burden of keystone pathogens
3 keystone pathogens that were measured all decreased3,*
- In a novel study, 11 individual pathogens were measured following treatment with either SRP + MM [ARESTIN] or SRP alone3
- At the 6-month periodontal maintenance, F nucleatum showed significantly greater decrease from baseline than with SRP alone, as did P intermedia, C rectus, and E corrodens3
- F nucleatum is a prominent orange complex bacteria that is central to interactions between gram-positive and gram-negative bacteria5
Cumulative salivary pathogens decreased in both groups3
However, larger decreases were observed in the SRP + MM [ARESTIN] group3
Limitations included the lack of a blinded examiner for clinical outcomes and the lack of patient-reported outcome measures.3
SELECT IMPORTANT SAFETY INFORMATION
- The safety and effectiveness of ARESTIN has not been established in immunocompromised patients or in those with coexistent oral candidiasis. Use with caution if there is a predisposition to oral candidiasis.
ARESTIN was studied in a randomized, open-label, controlled clinical trial comparing ARESTIN + SRP vs SRP alone. Subjects were 70 male and female adults ≥18 years old with a diagnosis of Stage II to Stage IV, Grade B periodontitis. Inclusion criteria were a minimum of 8 sites with a PD of ≥5 mm and 8 sites of BOP in any of the 4 quadrants. The primary outcome measure was an assessment of the adjunctive effects of ARESTIN on PD, CAL, BOP, and GI compared with SRP alone. The secondary outcome measure was a determination of the relative numbers of 11 periodontal pathogens in saliva after treatment with ARESTIN + SRP compared with SRP alone.




“After 1 month of treatment, patients receiving SRP plus minocycline microspheres had a significantly greater mean reduction in PD when compared with vehicle and control groups (P <0.001).”1

BOP, bleeding on probing; CAL, clinical attachment loss; GI, gingival index; MM, minocycline microspheres; PD, pocket depth; SRP, scaling and root planing.
REFERENCES: 1. Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72(11):1535-1544. doi:10.1902/jop.2001.72.11.1535 2. ARESTIN® (minocycline hydrochloride) microspheres, 1 mg. Prescribing Information. OraPharma; Bridgewater, NJ. 3. Arnett MC, Chanthavisouk P, Costalonga M, Blue C, Evans MD, Paulson DR. Effect of scaling and root planing with and without minocycline HCl microspheres on periodontal pathogens and clinical outcomes: a randomized clinical trial. J Periodontol. Published online May 16, 2023. doi:10.1002/JPER.23-0002 4. Goodson JM, Gunsolley JC, Grossi GG, et al. Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. J Periodontol. 2007;78(8):1568-1579. doi:10.1902/jop/2007.060488 5. Signat B, Roques C, Poulet P, Duffaut D. Role of Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol. 2011;13(2):25-36. doi:10.21775/cimb.013.025
IMPORTANT SAFETY INFORMATION AND INDICATION
- ARESTIN® (minocycline HCl) is contraindicated in any patient who has a known sensitivity to minocycline or tetracyclines. Hypersensitivity reactions and hypersensitivity syndrome that included, but were not limited to anaphylaxis, anaphylactoid reaction, angioneurotic edema, urticaria, rash, eosinophilia, and one or more of the following: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis may be present. Swelling of the face, pruritus, fever and lymphadenopathy have been reported with the use of ARESTIN. Some of these reactions were serious. Post-marketing cases of anaphylaxis and serious skin reactions such as Stevens Johnson syndrome and erythema multiforme have been reported with oral minocycline, as well as acute photosensitivity reactions.