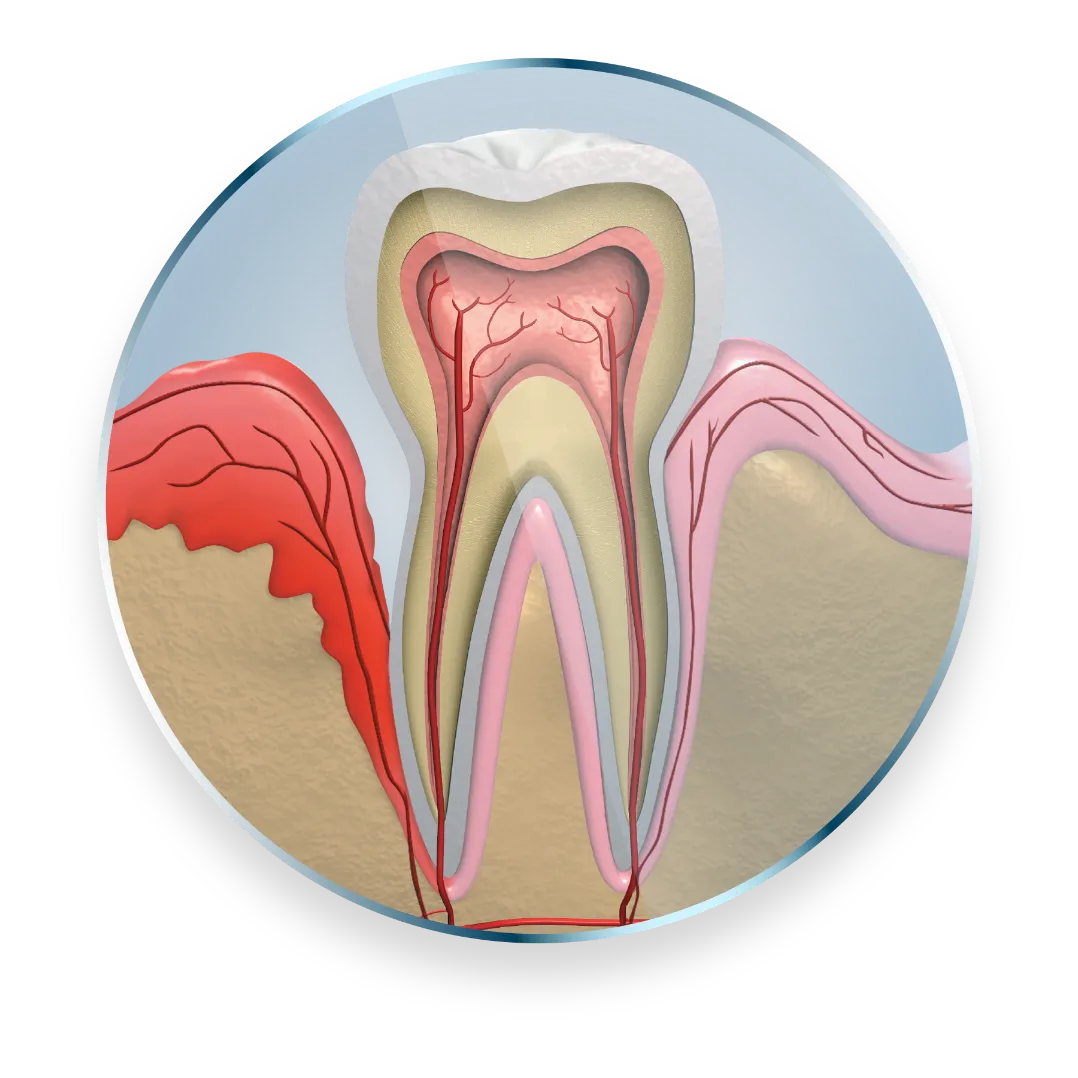
Treat a site-specific disease with a targeted approach
ARESTIN is the only FDA-approved locally applied antibiotic for use with SRP as part of
periodontal disease management.1
Subgingival sustained-release
product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer1
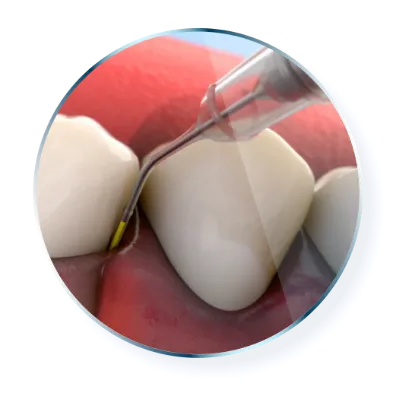
Each unit-dose cartridge
delivers minocycline hydrochloride equivalent to 1 mg of minocycline free base1
Bacteriostatic
and exerts its antimicrobial activity by inhibiting protein synthesis1

Subgingival sustained-release
product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer1

Each unit-dose cartridge
delivers minocycline hydrochloride equivalent to 1 mg of minocycline free base1

Bacteriostatic
and exerts its antimicrobial activity by inhibiting protein synthesis1

SRP, scaling and root planing.
REFERENCES: 1. ARESTIN® (minocycline hydrochloride) microspheres, 1 mg. Prescribing Information. OraPharma; Bridgewater, NJ. 2. Van der Weijden GA, Dekkers GJ, Slot DE. Success of non-surgical periodontal therapy in adult periodontitis patients: a retrospective analysis. Int J Dent Hygiene. 2019;3090317. doi:10.1111/idh.12399
IMPORTANT SAFETY INFORMATION AND INDICATION
- ARESTIN® (minocycline HCl) is contraindicated in any patient who has a known sensitivity to minocycline or tetracyclines. Hypersensitivity reactions and hypersensitivity syndrome that included, but were not limited to anaphylaxis, anaphylactoid reaction, angioneurotic edema, urticaria, rash, eosinophilia, and one or more of the following: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis may be present. Swelling of the face, pruritus, fever and lymphadenopathy have been reported with the use of ARESTIN. Some of these reactions were serious. Post-marketing cases of anaphylaxis and serious skin reactions such as Stevens Johnson syndrome and erythema multiforme have been reported with oral minocycline, as well as acute photosensitivity reactions.